From LifeArcReviewed by Olivia Frost
This article and associated images are based on a poster originally authored by Nikita Kathuria, Annabelle Herrington-Symes, Elisha Moran, Han Yin, Kathryn Armour, Michael Marshall, Nathan Robertson Paul Homes, Sarah Davies, Sophie Snow and Vincentius Aji Jatikusumo and presented at ELRIG Drug Discovery 2025 in affiliation with LifeArc.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Who is LifeArc?
- LifeArc is a self-financing medical research charity and guided by purpose, vision and mission to deliver patient impact.
- We specialise in early-stage translation and partner with academics, industry, charities and patient groups.
Antibody engineering platforms
- Antibody humanisation (including VHHs and new modalities) is one of our capabilities.
- In addition, we have experience in Fc engineering and producing multivalent and bispecific antibodies. These techniques can be applied to humanised antibodies and other antibodies from our discovery platforms.
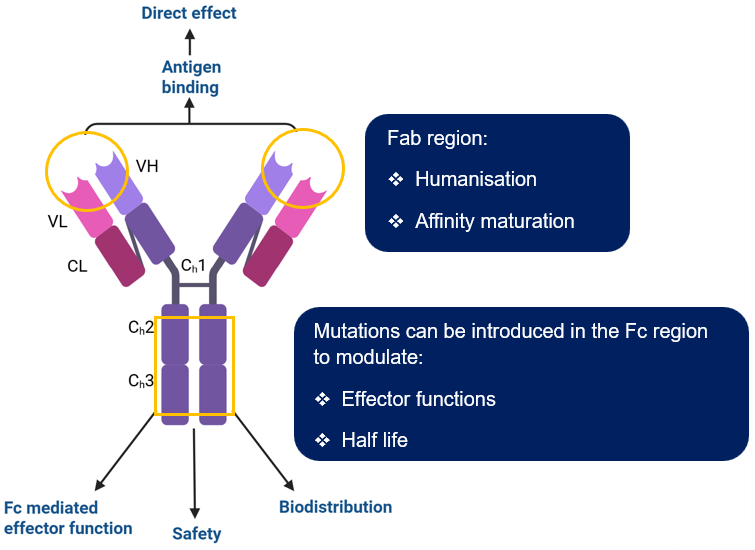
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
Introduction to effector functions
An antibody activates the immune system's cellular and cytoplasmic defence to combat a pathogen. This occurs via interactions of the Fc (CH2-CH3) region to activate three effector function pathways and impacts half-life of antibody (Figure 1). These can be modulated via several Fc mutations to achieve bespoke effects, specific to project needs.
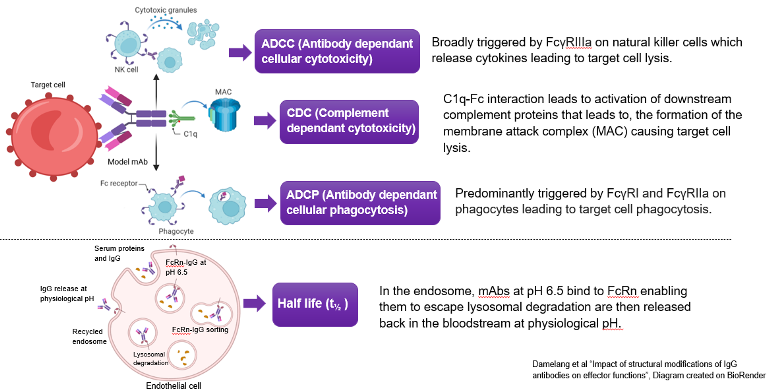
Figure 1. All three effector function pathways and half-life mechanism triggered by the Fc region. Image Credit: Damelang et al "Impact of structural modifications of IgG antibodies on effector functions", Diagram created on BioRender
Aim
Bridge the gap in literature by producing combined Fc effector function and half-life extension mutations to investigate how these may affect the biophysical/functional properties of the therapeutic mAb being developed.
Production of Trastuzumab Fc mutant antibodies
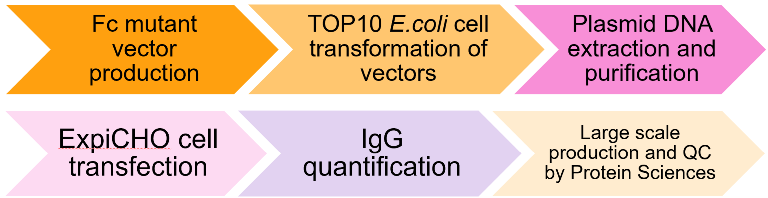
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
Biophysical developability assessment
All Phase III mutants have been developed on the Trastuzumab backbone. These were characterised by a range of biophysical and functional assays.
Q combined mutations have also reported a lower melting temperature (Tm).
Mutation D is hydrophobic and introduces hydrophobicity in Fc combination mutants.
Table 1. shows all the biophysical and functional assays performed on the Trastuzumab mutations. Source: ELRIG (UK) Ltd.
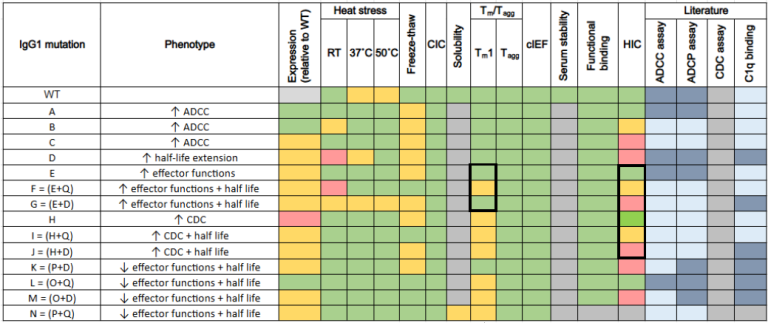
* Q is a half-life extension tested in previous phases of the effector function toolbox.
* R is a CDC increasing mutation, also tested in previous phases.
* P is a mutation that reduces all effector functions, also tested in previous phases.
Characterisation assays
Trastuzumab Fc mutants were screened for their functional activity on the following assays:
- HER2 binding ELISA: to see if the Fc mutants retain binding to their target.
- ADCC/ADCP reporter assays: Surrogate assays to investigate Fc mutants effect on ADCC/ADCP activity using a luciferase signal.
- C1q binding ELISA: a sandwich ELISA was carried out to test the Fc mutants binding to C1q to indicate the expected CDC activity.
Note: Refer to Table 1 for Fc mutations nomenclature, described in the below results.
HER2 ELISA
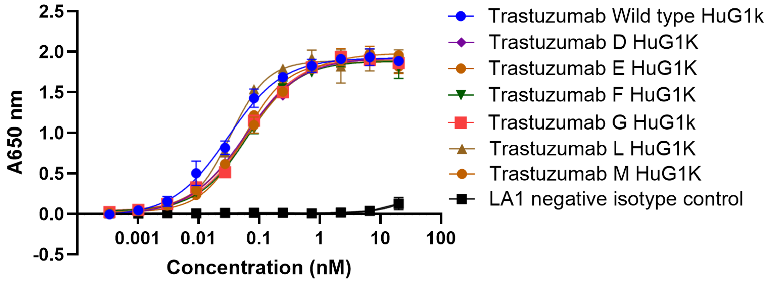
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
- All Trastuzumab Fc mutants retained binding to their target, HER2.
- There are some subtle differences seen in their binding activity.
C1q ELISA
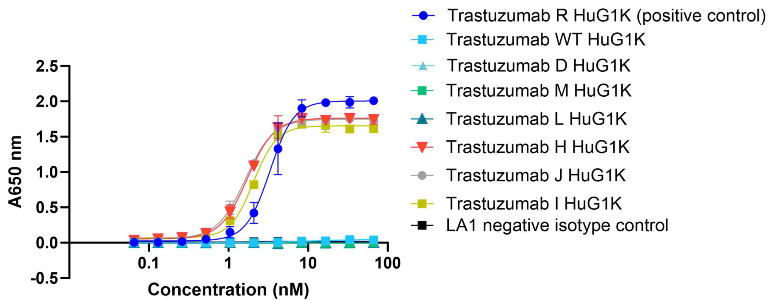
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
- Combined reducing effector function mutations L and M, half-life mutation D, WT all overlay the negative isotype control hence show no C1q binding.
- Mutation J demonstrated the same high C1q binding plateau to its single Fc mutation H, higher than WT but plateaus lower than positive control mutation R.
- Whereas mutation I (combined with a different half-life extension mutation to mutation J, Table 1) showed a slight reduced C1q binding plateau relative to its single Fc mutation H.
ADCC reporter assay
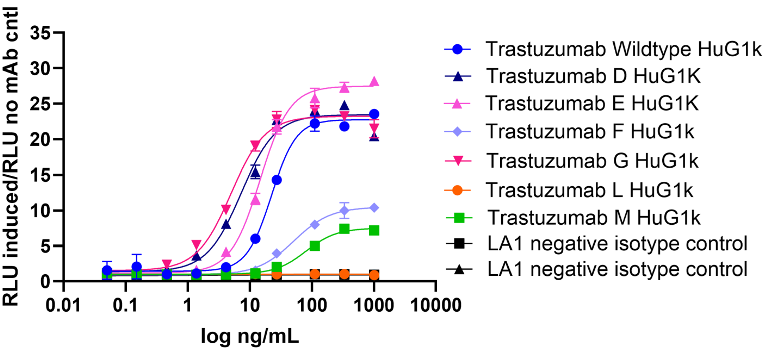
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
- Single Fc mutation E, has an increased plateau and therefore higher ADCC activity than WT.
- Combination mutation G resulted in an increase in ADCC activity relative to mutation E.
- Mutation F (E plus another half-life extension mutation, Table 1) shows a lower plateau and reduced ADCC activity compared to mutation E alone.
- Mutation D (half-life extension) rescues mutation M’s ADCC activity, whereas not seen in mutation L.
ADCP reporter assay
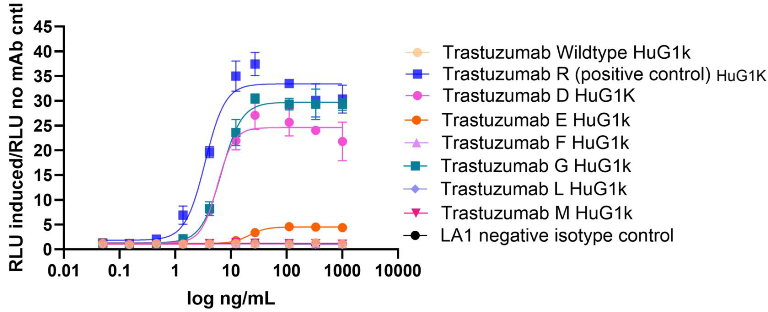
Image Credit: Image courtesy of Nikita Kathuria et al., in partnership with ELRIG (UK) Ltd.
- Mutation D has an increased ADCP activity, evident by a higher plateau level.
- Mutation E has a significantly lowered plateau and displayed a reduced ADCP activity compared to the positive control mutation R.
- Mutation G (E combined with D, Table 1) plateaus higher and increases ADCP activity relative to E.
- No ADCP activity observed for mutation F (combined with a different half-life mutation), or with mutations M and L that reduce effector functions.
Conclusions
- 8 Trastuzumab Fc effector function and half-life extension combined mutations have been added to the effector function toolbox.
- We have also investigated afucosylation of some single Fc mutations.
- All Trastuzumab Fc mutants retain their ability to bind to their target (HER2).
- Mutation D (half-life increasing mutation) has shown to increase ADCC and ADCP activity in reporter assays.
- Q combined mutations (Table 1) have lower melting temperatures (expected from literature) and mutation D, and its combined mutations introduce hydrophobicity.
Acknowledgements
We would like to thank the Analytical Sciences and Protein sciences team in Biologics Discovery & Development department, as well as members of Molecular and Cellular Pharmacology for their contribution to this work.
About LifeArc
LifeArc® is a medical research charity making life science life changing, transforming promising life science ideas into medical breakthroughs that change patients’ lives. We are self-funding and specialize in early-stage translation – advancing lab-based scientific discoveries to a point at which they can be developed into the next generation of diagnostics, treatments and cures. We have been doing this for more than 25 years and our work has resulted in a diagnostic for antibiotic resistance and four licensed medicines. This includes Keytruda®(cancer), Actemra® (rheumatoid arthritis), Tysabr® (multiple sclerosis) and Entyvio® (Crohn’s disease) and a test for antimicrobial resistance.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Jan 6, 2026