This article is based on a poster originally authored by Xiaohui Chi, Rong Zhang, Jiafu Mu, Tong Li, Xinhao Xie, Yujie Duan, Chunyan Han and Man Xu.
Introduction
Drug binding in primary hepatocytes is an important test for in vitro ADME research. To effectively detect drug binding in hepatocytes, it is necessary to deactivate their metabolic activity.
This has been accomplished traditionally by using heat inactivation, enzyme inhibitors, or freeze-thaw cycles. This study used these three ways to assess the unbound percentage of ten commercial drugs in hepatocytes and determined which method was most suited for routine testing.
Methods
- Heat inactivation method: The heat inactivation approach involves immersing hepatocyte suspensions in boiling water for five minutes.
- Enzyme Inhibitor method: The enzyme inhibitor approach involved incubating hepatocyte suspensions at 37 °C for one hour with 1-ABT (1 mM), then adding salicylamide (1.5 mM) and incubating for another five minutes.
- Freeze-Thaw method: Hepatocyte suspensions were frozen at -80 °C and thawed at 4 °C. The freeze-thaw cycle was repeated three times. The procedure was tried with either Williams' E Medium (supplemented with 1 % GlutaMAX) or Leibovitz's L-15 medium.
% fuhepatocyte of commercial drugs in rat hepatocytes
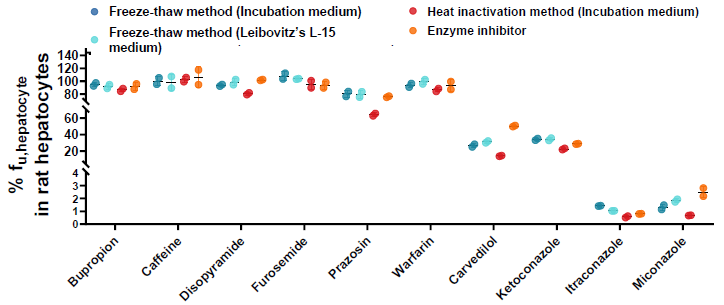
Image Credit: Pharmaron
By comparing the percentage unbound (%) and the three approaches, it was discovered that, for the majority of substances, the three methods produced comparable findings.
For the chemical carvedilol, however, the freeze-thaw approach increased the stability from 26.43 % to 73.93 % when compared to the Enzyme Inhibitor method, demonstrating that the freeze-thaw method has a higher enzyme inactivity ability.
% fuhepatocyte of commercial drugs in human hepatocytes
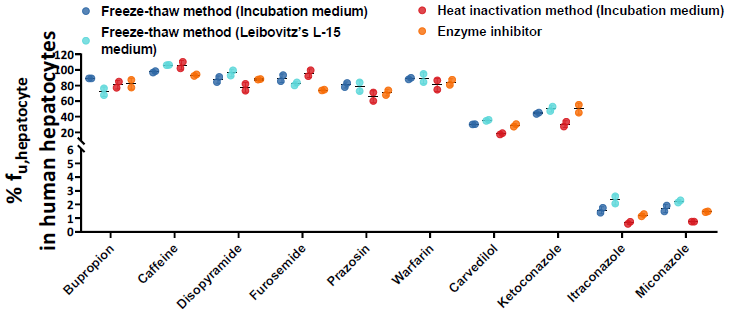
Image Credit: Pharmaron
For the majority of substances, the three approaches produced comparable results, just as they did for rats. However, for the chemical carvedilol, the freeze-thaw approach increased its recovery from 54.84 % to 82.16 % when compared to the Enzyme Inhibitor method, demonstrating that the freeze-thaw method has a better enzyme inactivation ability.
% fuhepatocyte in rat hepatocytes by different methods
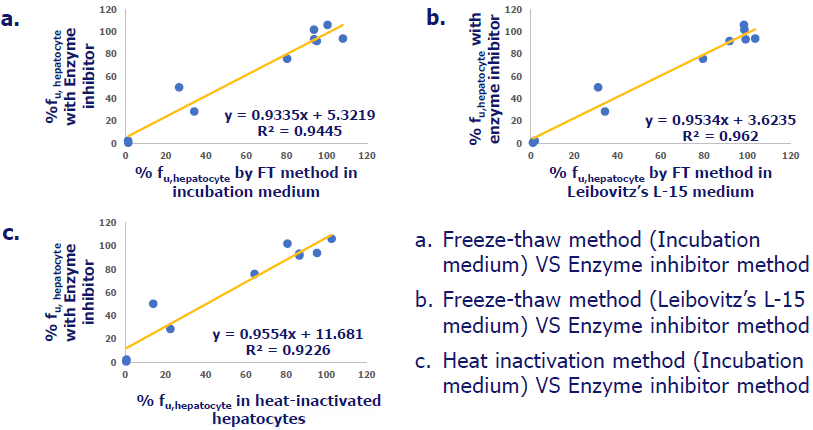
Image Credit: Pharmaron
% fuhepatocyte in human hepatocytes by different methods
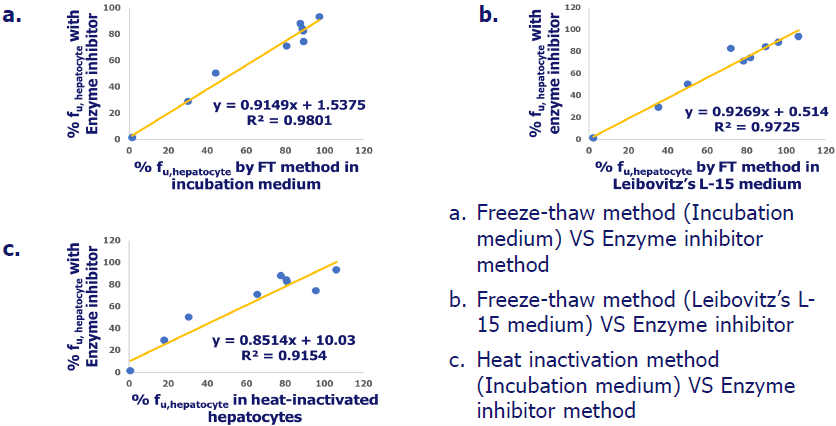
Image Credit: Pharmaron
The morphology of human hepatocytes after different treatments
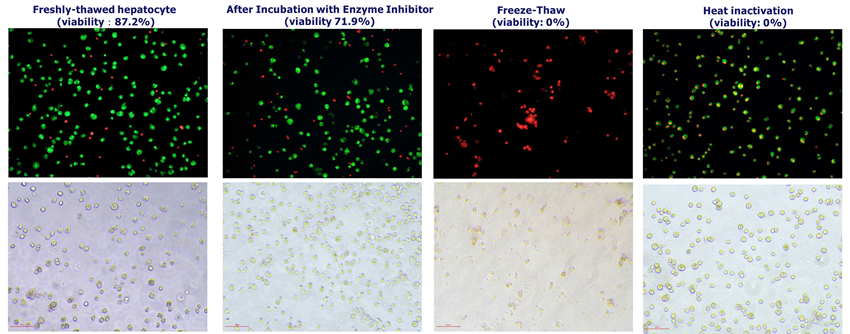
Image Credit: Pharmaron
- Enzyme inhibitors: 1-ABT (incubated for 1 hour) and salicylamide (5 minutes).
- Freeze-thaw cycle: ① -80 °C 1 h, 4 °C 2 h; ② -80 °C 1 h, 4 °C 2 h; ③ -80 °C 1 h, 4 °C 2 h.
- Heat inactivation method: boil for five minutes.
- The fluorescence pictures of hepatocytes were labeled with acridine orange (AO) and propidium iodide (PI).
Green cells indicate viability
Conclusion
For most substances, the Enzyme Inhibitor and Freeze-Thaw techniques produced similar binding results.
For carvedilol, the FreezeThaw approach significantly improved metabolic stability (26.43 % vs. 73.93 % after 4 hours), and microscopy revealed higher membrane rupture, indicating more efficient inactivation.
Overall, the Freeze-Thaw method provides practical benefits such as better inactivation for some medicines, streamlined processes, and clear morphological evidence, making it a more convenient and trustworthy option for routine in vitro ADME research.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development, and manufacturing service capabilities throughout the entire drug discovery, preclinical, and clinical development process across multiple therapeutic modalities, including small molecules, biologics, and CGT products. With over 17,000 employees and operations in China, the U.S., and the U.K., Pharmaron has an excellent track record in the delivery of R&D solutions to its partners in North America, Europe, Japan, and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Jan 9, 2026